Introduction
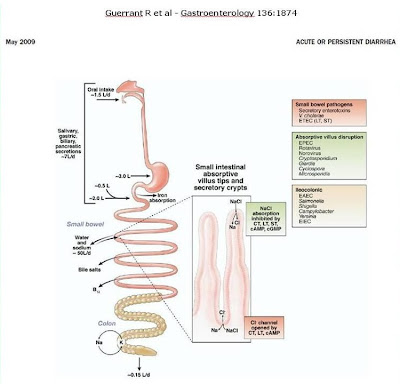
Diarrhea is still responsible for high rates of death in children worldwide. Diarrhea is the third cause of death (16%) among children under five years of age, after neonatal causes (37%) and pneumonia (17%) despite the decline of the mortality rates that have been observed since the 80`s (1) (Figure 1).
Figure 1- Schematic representation of the most important enteropathogenic agents and their local area of action.
In 2008, diarrheal disease responded for 15%, 1,336 million deaths of the estimated 8,795 million deaths in children younger than 5 years of age, around the world (2) (Figure 2).
Figure 1- Schematic representation of the most important enteropathogenic agents and their local area of action.
In 2008, diarrheal disease responded for 15%, 1,336 million deaths of the estimated 8,795 million deaths in children younger than 5 years of age, around the world (2) (Figure 2).
Figure 2
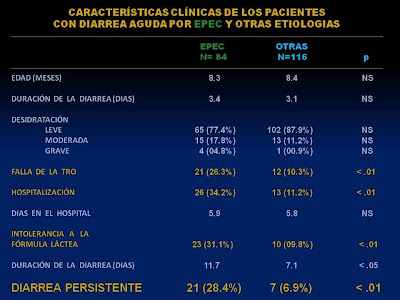
In our experience, studying 200 patients under one year of age suffering from acute diarrhea, we detected that when some strains of enteropathogenic Escherichia coli were isolated in the stools the evolution to persistent diarrhea (PD) reached 28.4% of the cases versus 6.9% when the process was due to another enteropathogenic agent (3) (Figure 3).
In developing countries, over 50% of the deaths due to diarrhea are associated to the perpetuation of the diarrheic syndrome (4). The majority of deaths occur in children of early age living in the rural regions of developing countries, where there is a lack of adequate sanitary conditions (5). Repeated episodes of acute diarrhea in the first year of life usually lead to intestinal malabsorption of the nutrients of the diet and consequently malnutrition. Considering that the perpetuation of the diarrheic episode in the majority of the cases occurs during a critical developmental period, both physical and intellectual, it could lead to severe damage of the rhythm of growth, the cognitive and intellectual functions, and in the future performance at school as well as an increasing morbi-mortality due to other diseases (6-7-8) (Figure 4).
Figure 4- Patient in hypovolemic shock due to infection of enteropathogenic Escherichia coli O119, that caused severe damage to the small bowel mucosa leading to malabsorption of the nutrients including glucose malabsorption.
Figure 3
In developing countries, over 50% of the deaths due to diarrhea are associated to the perpetuation of the diarrheic syndrome (4). The majority of deaths occur in children of early age living in the rural regions of developing countries, where there is a lack of adequate sanitary conditions (5). Repeated episodes of acute diarrhea in the first year of life usually lead to intestinal malabsorption of the nutrients of the diet and consequently malnutrition. Considering that the perpetuation of the diarrheic episode in the majority of the cases occurs during a critical developmental period, both physical and intellectual, it could lead to severe damage of the rhythm of growth, the cognitive and intellectual functions, and in the future performance at school as well as an increasing morbi-mortality due to other diseases (6-7-8) (Figure 4).
Figure 4- Patient in hypovolemic shock due to infection of enteropathogenic Escherichia coli O119, that caused severe damage to the small bowel mucosa leading to malabsorption of the nutrients including glucose malabsorption.
In the early 80`s in Brazil, several interventions of universal characteristics introduced by the Brazilian Public Health System including important improvements in the sanitary conditions and in the quality of water led to a dramatic decrease, in up to 90%, of the mortality rates in childhood due to diarrhea (9). Confirming these data Maranhão et al (10) conducted a case-control study in Natal, Rio Grande do Norte, in the northeast region of Brazil, including 206 infants under 2 years of age with acute diarrhea (103 patients) to evaluate the potential risk factors for persistence of diarrhea after one month of follow-up. The most frequent enteropathogens found in the stools in the infants with acute diarrhea were rotavirus (36%), enteropathogenic Escherichia coli (EPEC) (11.6%) and Shigella (11.6%). Only 5.2% of the patients showed persistence of the diarrhea. These results clearly demonstrated that implementation of vertical programs and long-term horizontal approaches can make the fourth Millennium Development Goal – to reduce by two-thirds, between 1990 and 2015, the under-five mortality rate – achievable (11).
Definition
PD was defined by the World Health Organization (WHO) in 1987 as “as diarrheal episode of presumed infectious etiology that begins acutely, but has an unusually long duration, lasting more than 14 days,” leading to a deterioration of the nutritional status and a substantial risk of death (Figure 5).
The term does not include chronic or recurrent diarrheal disorders such as tropical sprue, celiac disease, cystic fibrosis, or other hereditary diarrheal disorders (12). Although, these other clinical entities were not included in the definition of PD by WHO experts, considering the present knowledge about the high prevalence of celiac disease around the world, it should be brought to attention as an important cause of protracted diarrhea that may lead to malnutrition, even in the developing countries (13).
Figure 5
The term does not include chronic or recurrent diarrheal disorders such as tropical sprue, celiac disease, cystic fibrosis, or other hereditary diarrheal disorders (12). Although, these other clinical entities were not included in the definition of PD by WHO experts, considering the present knowledge about the high prevalence of celiac disease around the world, it should be brought to attention as an important cause of protracted diarrhea that may lead to malnutrition, even in the developing countries (13).
Etiopathogenic aspects
The enteric infections occur as a consequence of high levels of environmental contamination due to the lack of sanitary conditions and the access to potable water associated to poor personal hygiene (5). Several different enteropathogenic agents can cause acute diarrhea in children. The agents that are isolated in the stools in children with PD are not always the same ones found in the acute phase of the episode. This finding suggests that a potential secondary infection may assume a relevant role in the persistence of the diarrheic process (14). Moreover, it has also been reported the isolation of multiple enteropathogenic agents in the stools of children with PD (15). The most frequent enteropathogenic microorganisms isolated from the stools of children with PD in several different centers around the world are listed in Table1 modified from Bhutta (16).
Table 1 - Pathogens associated with Persistent Diarrhea
Bacteriae
Enteroaggregative Escherichia coli
Enteropathogenic Escherichia coli
Campylobacter
Salmonella
Shigella spp
Clostridium difficile
Parasites
Giardia lamblia
Blastocystis hominis1
Cryptosporidium spp.1
Entamoeba histolytica
Cyclospora cayetanensis1
Enterocytozoon bieneusi (Microsporidium spp)1
1 - Especially associated with HIV infections
Bacteriae
Enteroaggregative Escherichia coli
Enteropathogenic Escherichia coli
Campylobacter
Salmonella
Shigella spp
Clostridium difficile
Parasites
Giardia lamblia
Blastocystis hominis1
Cryptosporidium spp.1
Entamoeba histolytica
Cyclospora cayetanensis1
Enterocytozoon bieneusi (Microsporidium spp)1
1 - Especially associated with HIV infections
PD represents the final consequence of a variety of injuries suffered by the child, who becomes prone to frequent and severe episodes of diarrhea due to a combination of several factors depending on the host and the undesirable effect of a prevalent environmental contamination. These episodes usually occur in children under 3 years of age (17). The protein-energy malnutrition is seen as the major risk factor for the persistence of the diarrheic process (6). Furthermore, other determinants must also be considered, such as a recent episode of acute diarrhea (18), zinc deficiency (19), lack of breastfeeding (1) male sex (20) infection due to enteropathogenic and enteroaggregative Escherichia coli strains, Cryptosporidium (21) and past history of intrauterine growth retardation (20) (Figure 6).
Figure 6- Enteroaggregative Echerichia coli strain in HeLa cels culture producing the stacked brick pattern of adhesion.
Figure 6- Enteroaggregative Echerichia coli strain in HeLa cels culture producing the stacked brick pattern of adhesion.
Pathophisiology
There are multiple and complex pathophisiological mechanisms that may be involved and determine the perpetuation of an acute diarrheic episode. Small bowel injury has been incriminated as the central mechanism in the persistence of diarrhea (22,23). The status of the mucosal permeability barrier and the ability of the host in the clearance of the enteropathogenic agent have a direct influence on the persistence of these lesions (20).
It is well known that some enteropathogenic agents cause diarrhea by damaging the small bowel mucosa and/or through the secretion of enterotoxins that act on the enterocytes triggering the secretion of water and electrolytes, and even by the production of some cytotoxins that induce cell damage (24,25) (Figures 7-8-9-10).
Figure 7- Small bowel biopsy of the patient with persistent diarrhea (see Figure 4 above) showing severe vilous athrophy and the presence of clusters of bacteriae titghly adhered to the enterocytes.
Figure 8- Electron microfotography of the enterocyte showing an enteropathogenic Escherichia coli strain leading to dissolution of the microvilli and firm adherence to the apical portion of the enterocyte.
Figure 9- Electron microfotography of the enterocyte showing an enteropathogenic Escherichia coli strain adhered to the apical portion of the enterocyte inducing the classical lesion in pedestal.
Figure 10- A close vision of the patient of multiple food intolerance during the initial phase of the hospitalization.
Figure 7- Small bowel biopsy of the patient with persistent diarrhea (see Figure 4 above) showing severe vilous athrophy and the presence of clusters of bacteriae titghly adhered to the enterocytes.
Figure 8- Electron microfotography of the enterocyte showing an enteropathogenic Escherichia coli strain leading to dissolution of the microvilli and firm adherence to the apical portion of the enterocyte.
Figure 9- Electron microfotography of the enterocyte showing an enteropathogenic Escherichia coli strain adhered to the apical portion of the enterocyte inducing the classical lesion in pedestal.
Figure 10- A close vision of the patient of multiple food intolerance during the initial phase of the hospitalization.
EAEC and EPEC, among the several other agents, are usually the most frequent enteropathogenic agents isolated in the stools of infants with DP. The mechanisms of EAEC and EPEC infections are also the most detailed known and studied in the medical literature (24,25,26).
Enteroaggregative Escherichia coli (EAEC) infection represents an important cause of persistent diarrhea in developing countries (25). The investigation of the role of the EAEC strains in the persistence of the diarrhea has shown the ability of this microorganism to adhere both to the small bowel and the colonic mucosa. Hicks et al. (26) examined the interaction between EAEC and the human intestine using the in vitro organ culture model from biopsies obtained from infants with diarrhea. The EAEC strains were able to adhere to the jejunal, ileal and colonic mucosa and most of the bacteria were associated with the mucus layer above the intestinal mucosa and few of them were found in close association with the mucosal surface. Andrade et al. (27) studying the interaction of EAEC strains, isolated from the stools of infants with PD, with the human fragments of ileal and colonic mucosa in vitro, utilizing the transmission and scanning electronic microscopy, showed the occurrence of bacterial aggregates colonizing and provoking cytotoxic effects in the ileal and colonic mucosa (Figures 11-12).
Aggregative adherent fimbriae that are encoded on a 60 mda virulence plasmid called paa create a mucus biofilm that seems responsible for the small bowel damage leading to malabsorption of the nutrients and persistence of the diarrhea (28).
In contrast to the limited importance of EPEC in developed countries, EPEC is a major cause of infant diarrhea in developing countries (29) and in many cases responsible for PD (30). Proximal small intestinal mucosal biopsy specimens, but not always, show intimately adherent bacteria and the classic attaching and effacing (A/E) histopathology (31).
Cantey and Blake (32) were the first authors to describe this new pattern of adherence of an EPEC strain in rabbits, which was characterized by adherence of bacteria to the apical portion of the enterocyte, with cuplike pedestal formation and subsequent effacement of the brush border. This effect was designated by Moon et al. as attaching and effacing (A/E) (33). Rothbaum et al. (34) then reported on the adherence of EPEC O119:B14 associated with effacement of microvilli, leading to the formation of cuplike pedestal on enterocytes of infants with PD. The determinants of the A/E lesion have been localized to a large island of pathogenicity on EPEC chromosomes termed the locus of enterocyte effacement or LEE (35). Fagundes-Neto et al. (36) reported the presence of bacteria within the enterocytes from an infant with acute diarrhea that evolved to DP caused by EPEC O111:H2. Fagundes-Neto et al. (23) studied patients with DP in whom EPEC strains were isolated in the jejunal fluid secretion and in the stools utilizing the scanning electron microscopy. At low magnification (150X) most of the villi showed mild to moderate stunting, but on several occasions there was subtotal villus atrophy (Figure 13).
At higher magnification (7,500X) photomicrographs showed derangement of the enterocytes; on several occasions cell borders were not clearly defined, and very often microvilli were decreased in number and height; in some areas there was complete disappearance of the microvilli. Moreover, several baciliform micro-organisms tightly adhering to the enterocytes were seen, and lymphocytes and fat droplets were overlying the surface of the enterocytes as well. In half of the patients a mucous-fibrinoid pseudomembrane partially coating the enterocytes was observed. This mucus coating may hamper absorption of the nutrients of the diet due to mechanical block, thus leading to osmotic diarrhea and nutritional aggravation. This hypothesis may be supported by the finding of fat droplets accumulated on the apical surfaces of the enterocytes. These ultrastructural changes may arise from a combined interaction of the enteropathogenic agent that causes the A/E lesion associated with disarrangement of the digestive-absorptive enzyme system, leading to malabsorption of the nutrients (24). These pathophysiological events determine the onset of food intolerance that is responsible for perpetuation of diarrhea and nutritional aggravation (Figures 14-15-16).
Figure 11
Figure 12
Aggregative adherent fimbriae that are encoded on a 60 mda virulence plasmid called paa create a mucus biofilm that seems responsible for the small bowel damage leading to malabsorption of the nutrients and persistence of the diarrhea (28).
In contrast to the limited importance of EPEC in developed countries, EPEC is a major cause of infant diarrhea in developing countries (29) and in many cases responsible for PD (30). Proximal small intestinal mucosal biopsy specimens, but not always, show intimately adherent bacteria and the classic attaching and effacing (A/E) histopathology (31).
Cantey and Blake (32) were the first authors to describe this new pattern of adherence of an EPEC strain in rabbits, which was characterized by adherence of bacteria to the apical portion of the enterocyte, with cuplike pedestal formation and subsequent effacement of the brush border. This effect was designated by Moon et al. as attaching and effacing (A/E) (33). Rothbaum et al. (34) then reported on the adherence of EPEC O119:B14 associated with effacement of microvilli, leading to the formation of cuplike pedestal on enterocytes of infants with PD. The determinants of the A/E lesion have been localized to a large island of pathogenicity on EPEC chromosomes termed the locus of enterocyte effacement or LEE (35). Fagundes-Neto et al. (36) reported the presence of bacteria within the enterocytes from an infant with acute diarrhea that evolved to DP caused by EPEC O111:H2. Fagundes-Neto et al. (23) studied patients with DP in whom EPEC strains were isolated in the jejunal fluid secretion and in the stools utilizing the scanning electron microscopy. At low magnification (150X) most of the villi showed mild to moderate stunting, but on several occasions there was subtotal villus atrophy (Figure 13).
Figure 13
At higher magnification (7,500X) photomicrographs showed derangement of the enterocytes; on several occasions cell borders were not clearly defined, and very often microvilli were decreased in number and height; in some areas there was complete disappearance of the microvilli. Moreover, several baciliform micro-organisms tightly adhering to the enterocytes were seen, and lymphocytes and fat droplets were overlying the surface of the enterocytes as well. In half of the patients a mucous-fibrinoid pseudomembrane partially coating the enterocytes was observed. This mucus coating may hamper absorption of the nutrients of the diet due to mechanical block, thus leading to osmotic diarrhea and nutritional aggravation. This hypothesis may be supported by the finding of fat droplets accumulated on the apical surfaces of the enterocytes. These ultrastructural changes may arise from a combined interaction of the enteropathogenic agent that causes the A/E lesion associated with disarrangement of the digestive-absorptive enzyme system, leading to malabsorption of the nutrients (24). These pathophysiological events determine the onset of food intolerance that is responsible for perpetuation of diarrhea and nutritional aggravation (Figures 14-15-16).
Figure 14
Figure 15
Figure 16
Bacterial overgrowth in the small bowel lumen of the colonic microflora may occur in up to 68% of the patients with PD and it is another factor that will perpetuate the damage of the intestinal mucosa (23). It is particularly associated with anaerobic bacteria such as Veillonella and Bacteroides species and predisposes to intestinal mucosal injury (37). Pathologic changes occur due to the ability of the anaerobic bacteria to induce deconjugation and 7α-dehydroxylation of the primary bile acids cholic and chenodeoxycholic acid, converting them into their respective secondary bile acids (deoxycholic and lithocholic acid), which are highly damaging to the jejunal mucosa provoking decrease in the absorption surface, and functional lesions with deficiency of enterokinase (38) and of the ATPase (Na+K+) enzyme (39) (Figure 17).
Figure 17
When present in the intestinal lumen, these secondary, unconjugated bile acids induce water and sodium secretion and glucose malabsorption, and can also lead to breakdown the intestinal permeability barrier, facilitating the penetration of potentially allergenic macromolecules (food proteins, intact or partially hydrolyzed), leading as a consequence allergy to the proteins of the diet (cow milk, soybean protein) (40).
The presence of secondary and unconjugated bile salts in the small bowel prevents the formation of the mixed micelles, which play an essential role in ensuring solubilization of dietary fats. The consequent decrease of bile salts pool will lead, first, to malabsorption of fats of the diet, having as a result steatorrhea, which will deprive the patient from an important caloric offer (41). Secondly, the excretion of bile salts will induce the appearance of cholereic diarrhea, due to the direct toxic action of the bile salts on the colonic mucosa (42).
Finally, the synergistic effects of these pathophysiologic events become responsible for the perpetuation of the diarrheal process and for the aggravation of the nutritional status, with high risk of death.
1- Wardlaw T, Salama P, Brocklehurst C, Chopra M, Mason E. Diarrhoea: why children are still dying and what can be done. Lancet 2010; 375:870-2.
2- Black RE, Cousens S, Johnson S, Lawn JE, Rudan I, Bassani DG et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010; 375: 1969–87.
3- Fagundes-Neto, U; Scaletsky, ICA. The gut at war: the consequences of enteropathogenic Escherichia coli infection as a factor of diarrhea and malnutrition. São Paulo Med J 2000; 118:21-9.
4- Matters CD, Bernard C, Iburg KM, Inoue M, Fat DM, Shibuya K et al. Global burden of disease in 2002: data sources, methods and results. In Global programme on evidence for health policy discussion paper no. 54, (revised 2004). WHO Geneva; 2003:45.
5- Black, RE; Morris, SS; Bryce, J. Where and why are 10 million children dying every year? Lancet 2003; 361:2226-34.
6- Guerrant RL, Oriá RB, Moore, SR, Oriá MOB, Lima AAM. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev 2008; 66: 487–505.
7- Petri Jr WA, Miller M, Binder HJ, Levine MM, Dillinghan R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest 2008; 118:1277–90.
8- Moore SR, Lima NL, Soares AM, Oriá RB, Pinkerton RC, Barret LJ et al. Prolonged Episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gatroenterology 2010; 139:1156-64.
9- Victora CG. Mortalidade por diarréia: o que o mundo pode aprender com o Brasil? J Pediatr 2009; 85:3-5.
10- Maranhão HS, Medeiros MCC, Scaletsky ICA, Fagundes-Neto U, Moraes MB. The epidemiological and clinical characteristics and nutritional development of infants with acute diarrhoea, in North-eastern Brazil. Ann Trop Med Parasitol 2008; 102:357-65.
11- The United Nations Children's Fund (UNICEF). Countdown to 2015: maternal, newborn and child survival. Tracking progress in maternal, neonatal and child survival: the 2008 report. New York, NY: UNICEF; 2008.
12- Persistent diarrhoea in children in developing countries: Memorandum from a WHO meeting*. Bull of the WHO 1988; 66:709-717.
13- Oliveira RP, Sdepanian VL, Barreto JA, Cortez AJP, Carvalho FO, Bordin JO et al. High prevalence of celiac disease in Brazilian blood donor volunteers based on screening by IgA antitissue transglutaminase antibody. Eur J Gastroenterol Hepatol 2007;19:43-9.
14- Baqui AH, Sack RB, Black RE, Haider K, Hossain A, Alim ARMA et al. Enteropathogens associated with acute and persistent diarrhea in Bangladeshi children < 5 years of age. J Infect Dis 1992; 166:792-6.
15- Abba K, Sinfield R, Hart CA, Garner P. Pathogens associated with persistent diarrhea in children in low and middle income countries: systematic review. BMC Infect Dis 2009, 9:88:1-15.
16- Bhutta ZA. Persistent diarrhea in developing countries. Ann. Nestlé 2006;64:39-47.
17- Grimwood K & Forbes DA. Acute and Persistent Diarrhea. Pediatr Clin North America 2009; 56:1343-61.
18- García ALG, Martínez JD, Callejas NP, Herrera VC, Quintero MD. Persistent Diarrhea: bibliographical review. Mediciego 2006; 12:15-20.
19- Fischer Walker C L, Ezzati M, Black R E. Global and regional child mortality and burden of disease attributable to zinc deficiency, Eur J Clin Nutr. 2009; 63, 591–7.
20- Bhutta ZA, Ghishan F, Lindley K, Memon IA, Mittal S, Roads Jm. Persistent and chronic diarrhea and malabsorption: Working Group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 39 Suppl 2004; 2:S711.
21- Pawlowski SW, Warren CA, Guerrant R. Diagnosis and Treatment of Acute or Persistent Diarrhea. Gastroenterology 2009; 136: 1874-6.
22- Sullivan PB. Studies of the small intestine in persistent diarrhea and malnutrition: The Gambian experience. J Pediatr Gastroenterol Nutr 2002; 34:11-3.
23- Fagundes-Neto U, de Martini-Costa S, Pedroso MZ, Scaletsky IC. Studies of the small bowell surface by scanning electron microscopy in infants with persistent diarrhea. Braz J Med Biol Res 2000; 33:1437-42.
24- Law D. Adhesion and its role in the virulence of enteropathogenic E. coli. Clin Microbiol Rev 1994; 7:152-73.
25- Okhuysen PC & DuPont HL. Enteroaggregative Escherichia coli (EAEC): A cause of acute and persistent diarrhea of worldwide importance. J Infect Dis 2010; 202:503-5.
26- Hicks S, Candy DCA, Philips AD. Adhesion of Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun 1996; 64:4751-60.
27- Andrade JAB, Freymüller E, Fagundes-Neto U. Pathophysiology of enteroaggregative Escherichia coli infection: an experimental model utilizing transmission electron microscopy. Arq Gastroenterol 2010; 47:306-12.
28- Nataro JP, Deng Y, Meneval DR, German AL, Martin WC, Levine MM. Aggregative adherence fimbriae I of Enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human enterocytes. Infect Immun 1992; 60:2297-304.
29- Petri Jr. WA, Miller M, Binder HJ, Levine MM, Dillinghan R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest 2008; 118: 1277-90.
30- Ochoa Tj, Barletta F, Contreras C, Mercado E. New insights into the epidemiology of enteropathogenic Escherichia coli infection. Trans R Soc Trop Med Hyg 2008; 102:852-6.
31- Chen HD, Frankel G. Enteropathogenic Escherichia coli: unraveling pathogenesis. FEMS Microbiol Rev 2005; 29:83-98.
32- Cantey, JR & Blake RK. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis 1977; 135:454-62.
33- Moon HW, Whipp SC, Argenzio RA, Levine MM, Gianella RA. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun 1983; 41:1340-5.
34- Rothbaum R, MacAdams AJ, Gianella R, Partin JC. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology 1982; 83:441-54.
35- Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev 1998; 11:142-201.
36- Fagundes-Neto U, Freymuller E, Gatti MSV, Schmitz LG, Scatetsky I. Enteropathogenic Escherichia coli O111ab:H2 penetrates the small bowel epithelium in an infant with acute diarrhea. Acta Paediatr 1995; 84:453-5.
37- De Boisseau D; Chaussain M, Badoual J, Raymond J, Dupont C. Small bowel bacterial overgrowth in children with chronic diarrhea, abdominal pain or both. J Pediatr 1996; 147:410-1.
38- Lebenthal E, Antonowicz I, Shwachman H. The interrelationship of enterokinase and trypsin activities in intractable diarrhea of infancy, celiac disease, and intravenous alimentation. Pediatr 1975; 56:585-91.
39- Fagundes Neto U, Viaro T, Wheba J, Machado NL, Patrício FRS, Michalany J. Enteropatia tropical: alterações morfológicas e funcionais do intestino delgado e suas repercussões sobre o estado nutricional. Arq Gastroenterol 1981; 18:177-82.
40- Teichberg S, Fagundes-Neto U, Bayne MA, Lifshtz F. Jejunal macromolecular absorption and bile salt desconjugation in protein-energy malnourished rats. Am J Clin Nutr 1981; 34:1281-91.
41- Jonas A, Avigad S, Diver-Haber A, Katznelson D. Disturbed fat absorption following infectious gastroenteritis in children. J Pediatr 1979; 95:366-72.
42- Rosenberg IH, Solomons NW, Schneider RE. Malabsorption associated with diarrhea and intestinal infections. Am J Clin Nutr 1977; 30, 1248-53.
References
1- Wardlaw T, Salama P, Brocklehurst C, Chopra M, Mason E. Diarrhoea: why children are still dying and what can be done. Lancet 2010; 375:870-2.
2- Black RE, Cousens S, Johnson S, Lawn JE, Rudan I, Bassani DG et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010; 375: 1969–87.
3- Fagundes-Neto, U; Scaletsky, ICA. The gut at war: the consequences of enteropathogenic Escherichia coli infection as a factor of diarrhea and malnutrition. São Paulo Med J 2000; 118:21-9.
4- Matters CD, Bernard C, Iburg KM, Inoue M, Fat DM, Shibuya K et al. Global burden of disease in 2002: data sources, methods and results. In Global programme on evidence for health policy discussion paper no. 54, (revised 2004). WHO Geneva; 2003:45.
5- Black, RE; Morris, SS; Bryce, J. Where and why are 10 million children dying every year? Lancet 2003; 361:2226-34.
6- Guerrant RL, Oriá RB, Moore, SR, Oriá MOB, Lima AAM. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev 2008; 66: 487–505.
7- Petri Jr WA, Miller M, Binder HJ, Levine MM, Dillinghan R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest 2008; 118:1277–90.
8- Moore SR, Lima NL, Soares AM, Oriá RB, Pinkerton RC, Barret LJ et al. Prolonged Episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gatroenterology 2010; 139:1156-64.
9- Victora CG. Mortalidade por diarréia: o que o mundo pode aprender com o Brasil? J Pediatr 2009; 85:3-5.
10- Maranhão HS, Medeiros MCC, Scaletsky ICA, Fagundes-Neto U, Moraes MB. The epidemiological and clinical characteristics and nutritional development of infants with acute diarrhoea, in North-eastern Brazil. Ann Trop Med Parasitol 2008; 102:357-65.
11- The United Nations Children's Fund (UNICEF). Countdown to 2015: maternal, newborn and child survival. Tracking progress in maternal, neonatal and child survival: the 2008 report. New York, NY: UNICEF; 2008.
12- Persistent diarrhoea in children in developing countries: Memorandum from a WHO meeting*. Bull of the WHO 1988; 66:709-717.
13- Oliveira RP, Sdepanian VL, Barreto JA, Cortez AJP, Carvalho FO, Bordin JO et al. High prevalence of celiac disease in Brazilian blood donor volunteers based on screening by IgA antitissue transglutaminase antibody. Eur J Gastroenterol Hepatol 2007;19:43-9.
14- Baqui AH, Sack RB, Black RE, Haider K, Hossain A, Alim ARMA et al. Enteropathogens associated with acute and persistent diarrhea in Bangladeshi children < 5 years of age. J Infect Dis 1992; 166:792-6.
15- Abba K, Sinfield R, Hart CA, Garner P. Pathogens associated with persistent diarrhea in children in low and middle income countries: systematic review. BMC Infect Dis 2009, 9:88:1-15.
16- Bhutta ZA. Persistent diarrhea in developing countries. Ann. Nestlé 2006;64:39-47.
17- Grimwood K & Forbes DA. Acute and Persistent Diarrhea. Pediatr Clin North America 2009; 56:1343-61.
18- García ALG, Martínez JD, Callejas NP, Herrera VC, Quintero MD. Persistent Diarrhea: bibliographical review. Mediciego 2006; 12:15-20.
19- Fischer Walker C L, Ezzati M, Black R E. Global and regional child mortality and burden of disease attributable to zinc deficiency, Eur J Clin Nutr. 2009; 63, 591–7.
20- Bhutta ZA, Ghishan F, Lindley K, Memon IA, Mittal S, Roads Jm. Persistent and chronic diarrhea and malabsorption: Working Group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 39 Suppl 2004; 2:S711.
21- Pawlowski SW, Warren CA, Guerrant R. Diagnosis and Treatment of Acute or Persistent Diarrhea. Gastroenterology 2009; 136: 1874-6.
22- Sullivan PB. Studies of the small intestine in persistent diarrhea and malnutrition: The Gambian experience. J Pediatr Gastroenterol Nutr 2002; 34:11-3.
23- Fagundes-Neto U, de Martini-Costa S, Pedroso MZ, Scaletsky IC. Studies of the small bowell surface by scanning electron microscopy in infants with persistent diarrhea. Braz J Med Biol Res 2000; 33:1437-42.
24- Law D. Adhesion and its role in the virulence of enteropathogenic E. coli. Clin Microbiol Rev 1994; 7:152-73.
25- Okhuysen PC & DuPont HL. Enteroaggregative Escherichia coli (EAEC): A cause of acute and persistent diarrhea of worldwide importance. J Infect Dis 2010; 202:503-5.
26- Hicks S, Candy DCA, Philips AD. Adhesion of Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun 1996; 64:4751-60.
27- Andrade JAB, Freymüller E, Fagundes-Neto U. Pathophysiology of enteroaggregative Escherichia coli infection: an experimental model utilizing transmission electron microscopy. Arq Gastroenterol 2010; 47:306-12.
28- Nataro JP, Deng Y, Meneval DR, German AL, Martin WC, Levine MM. Aggregative adherence fimbriae I of Enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human enterocytes. Infect Immun 1992; 60:2297-304.
29- Petri Jr. WA, Miller M, Binder HJ, Levine MM, Dillinghan R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest 2008; 118: 1277-90.
30- Ochoa Tj, Barletta F, Contreras C, Mercado E. New insights into the epidemiology of enteropathogenic Escherichia coli infection. Trans R Soc Trop Med Hyg 2008; 102:852-6.
31- Chen HD, Frankel G. Enteropathogenic Escherichia coli: unraveling pathogenesis. FEMS Microbiol Rev 2005; 29:83-98.
32- Cantey, JR & Blake RK. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis 1977; 135:454-62.
33- Moon HW, Whipp SC, Argenzio RA, Levine MM, Gianella RA. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun 1983; 41:1340-5.
34- Rothbaum R, MacAdams AJ, Gianella R, Partin JC. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology 1982; 83:441-54.
35- Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev 1998; 11:142-201.
36- Fagundes-Neto U, Freymuller E, Gatti MSV, Schmitz LG, Scatetsky I. Enteropathogenic Escherichia coli O111ab:H2 penetrates the small bowel epithelium in an infant with acute diarrhea. Acta Paediatr 1995; 84:453-5.
37- De Boisseau D; Chaussain M, Badoual J, Raymond J, Dupont C. Small bowel bacterial overgrowth in children with chronic diarrhea, abdominal pain or both. J Pediatr 1996; 147:410-1.
38- Lebenthal E, Antonowicz I, Shwachman H. The interrelationship of enterokinase and trypsin activities in intractable diarrhea of infancy, celiac disease, and intravenous alimentation. Pediatr 1975; 56:585-91.
39- Fagundes Neto U, Viaro T, Wheba J, Machado NL, Patrício FRS, Michalany J. Enteropatia tropical: alterações morfológicas e funcionais do intestino delgado e suas repercussões sobre o estado nutricional. Arq Gastroenterol 1981; 18:177-82.
40- Teichberg S, Fagundes-Neto U, Bayne MA, Lifshtz F. Jejunal macromolecular absorption and bile salt desconjugation in protein-energy malnourished rats. Am J Clin Nutr 1981; 34:1281-91.
41- Jonas A, Avigad S, Diver-Haber A, Katznelson D. Disturbed fat absorption following infectious gastroenteritis in children. J Pediatr 1979; 95:366-72.
42- Rosenberg IH, Solomons NW, Schneider RE. Malabsorption associated with diarrhea and intestinal infections. Am J Clin Nutr 1977; 30, 1248-53.















Um comentário:
my heart really breaks seeing those children in dextrose fighting for their lives. :(
Postar um comentário